Thermodynamics and Types of Reactions
Before we get into learning about the mitochondrion, we need to know a little bit about the laws of thermodynamics.
First Law of Thermodynamics: energy cannot be created nor destroyed, only transferred.
- For example, when boiling a pot of water, the chemical or electrical energy is converted into heat. No energy is lost.
Second Law of Thermodynamics: entropy increases each time energy is transferred or transformed.
- For example, melting an ice cube will result in the (solid) ice cube turning into (liquid) water, which is a disordered state.
An oxidation reaction is a reaction with a loss of electrons. This is often found in biology as the loss of a hydrogen atom. Losing an electron (which is negative) means the molecule has become a little bit more positive, so in a reaction like this:
the oxidized molecule will typically be denoted with a '+'.
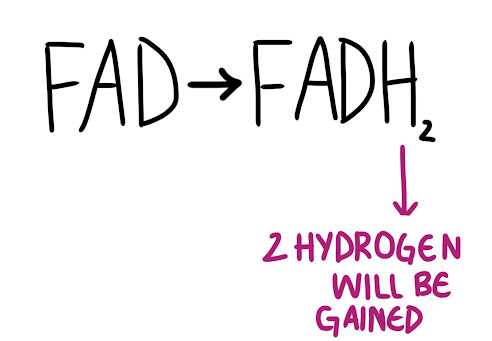
In a reduction reaction, an electron is gained by a molecule. A hydrogen atom will be added to a molecule.
An easy mnemonic to remember this is OIL RIG. Oxidation Is Losing, Reduction Is Gaining.
Both of the examples for these reactions (NADH and FADH2) are actually electron carriers (important for when we cover the mitochondrion!). This means that they usually store electrons (NAD has 1 electron, FAD has 2 electrons) to drive cellular processes.
That's it! Next up: The Mitochondrion, Part 1
(P.S. Apologies for the late post. I've been swamped with homework and college apps, but finally got around to making the next post) :D

finally another lesson!!
ReplyDeleteWhy do you need to know this for the mitochondria lesson?
ReplyDeleteBecause the mitochondria use FAD and NAD to produce energy! We'll cover it thoroughly :)
Deletewhy is a hydrogen aton used as an electron
ReplyDeleteHydrogen atoms consist of one proton and one electron. When a reaction happens, H molecules are transferred (which includes the electron). Instead of explicitly writing the electron separately to denote charge, an H is used instead.
DeleteHope that helped!