Enzymes
Now that we've learned what exactly a protein is, let's focus on a specific type of protein: an enzyme.
An enzyme is a biological catalyst that regulates chemical reactions. All enzymes have an active site, are specific (only work for their type of substrate) and are recycled. Examples include sucrase, lipase, protease and pepsin. Note that majority of enzymes will end in "-ase". If you see "-ase" at the end of a word, it is likely an enzyme.
A substrate is the substance that an enzyme acts upon. It is the reactant that binds to an enzyme.
You can guess what sort of substrate an enzyme will act upon by its name. Sucrase, for example, is made up of "sucrose" and "-ase" to get sucrase. Therefore, you can assume that sucrase is the enzyme for the substrate sucrose (type of sugar). Lipase is made up of "lipid" and "-ase" - therefore, lipase is the enzyme for lipids (fats).
Can you guess what substrate protease digests?
Yup! It's protein.
Can you guess what substrate pepsin digests? (trick question)
Pepsin actually also digests protein. However, while protease is found in the small intestine, pepsin is found in the stomach.
There are two types of enzymes: regulatory and non-regulatory. Non-regulatory enzymes only have an active site. Regulatory enzymes contain an active site, along with something called an allosteric site.
The active site of an enzyme is the enzyme's catalytic site. This is where a specific substrate will bind.
The allosteric site is an area on the enzyme where a non-substrate molecule will bind and affect the enzyme's activity. Sometimes, if something binds to the allosteric site, it can actually change the shape of the active site, so that either no substrate will be able to bind to the enzyme, or more substrates will be able to bind to the enzyme.
When the non-substrate particle binds to the allosteric site, the active site can be rendered nonfunctional. A chemical reaction can be inhibited by using regulatory enzymes, because if no substrate can bind to the active site, no product will be made. If a particle increases an enzyme's rate of reaction, it is an activator. If it decreases an enzyme's rate of reaction, it is an inhibitor.
Just remember:
Inhibitor = Inactive
Activator = Active
A common example of a non-substrate molecules is a metal ion, such as Mg, K, Ca, Fe, and Cu.
It's also important to note that some inhibitors can bind right into the active site and block substrates directly, instead of binding to the allosteric site (coming up).
Currently, there are 2 proposed models for how a substrate and enzyme fit together. Enzymes exhibit behaviors of both models, so both are correct despite being different.
The lock-and-key model is described as having a substrate that fits perfectly into the 3D structure of an enzyme's active site, much like how a key is molded exactly to fit the inside of a lock. In this model, there are H bonds between the substrate and enzyme.
In the induced fit model, an enzyme undergoes something called "conformational change": the substrate's binding to the active site causes the enzyme to change its shape for a tighter fit. So even if a substrate does not fit perfectly, the enzyme will mold itself to fit better. It will form H bonds, ionic bonds, VDW forces, and dipole-dipole forces. It will NOT form covalent bonds, peptide bonds, or disulphide bridges.
Inhibitors
There are 4 types of inhibitors: competitive, noncompetitive, irreversible, and feedback.
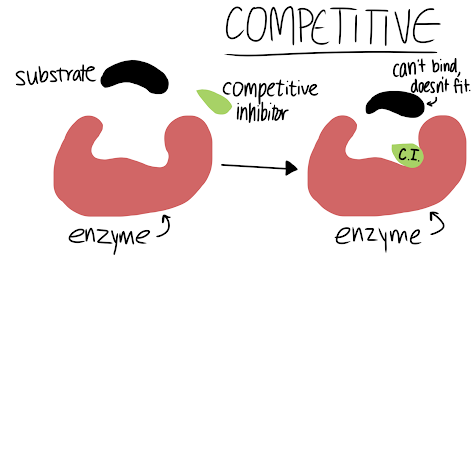
Competitive Inhibitor:
The inhibitor and substrate compete for the active site. If the inhibitor gets there first, the substrate cannot bind. No product will be made. An example is Antabuse (disulfiram), a medicine used to treat chronic alcoholism. It blocks the enzyme that breaks down alcohol.
Noncompetitive Inhibitor:
The inhibitor binds to the allosteric site and causes the enzyme's active site to change shape. The active site becomes inactive, and no product is made. It is therefore noncompetitive because it is not competing with the substrate.
Irreversible Inhibition:
Inhibitor permanently binds to the enzyme with strong covalent bonds. If it bonds permanently to the active site, it is competitive irreversible inhibition. If it bonds permanently to the allosteric site, it is noncompetitive irreversible inhibition. There is a permanent change to the shape of the enzyme, and it will never be able to bind with a substrate. This can be caused by toxins like cyanide, sarin, insecticides, and nerve gas.
A quick explanation before the next type of inhibition:
Negative feedback is a type of regulation where the end product of a reaction reduces the stimulus of that reaction. It is meant to bring homeostasis to the body; if you are hungry, you eat food to go back to being not hungry (homeostasis!).
Positive feedback is a type of amplification system. If you cut your skin, cells near the cut will give off signals that tell other cells to come and repair the cut, as well as signal even more cells to come.
Feedback Inhibition:
A type of negative feedback for chemical reactions. The inhibitor is actually a product from the enzyme's previous catalyzation. This product/inhibitor will bind to the allosteric site. This is useful in cases where a cell does not want unnecessary accumulation of a product.
You rocket!
(haha)
Next up is the structures and functions of carbs, lipids, and nucleic acids!









is atp energy used for binding
ReplyDeleteThe bonds between substrates and enzymes generally do not use ATP because the bonds are non-covalent (think: ionic or hydrogen bonds). However, ATP can act as an inhibitor in some cases - for example, in glycolysis (the lesson for that is not posted as yet, but you can check out resources on Khan Academy to learn more) when too much ATP is being made, it will follow feedback inhibition and stop more ATP from being made.
Deletethanks
DeleteDoes any enzyme use both competitive and noncompetitive?
ReplyDeleteIt's definitely possible! This is called mixed inhibition. They don't necessarily happen at the same exact time though.
Delete