Proteins, Monomers, and Polymers
We start off with monomers! A monomer is a type of molecule that has the ability to chemically bond with other monomers to create a chain. These include animo acids and glucose. Think of them like individual building blocks - you can connect them together to form a complete structure.
The chain we've made with these monomers is called a polymer - literally, just a chain of monomers. Polymers include cellulose and enzymes (a type of protein).
A protein is a polymer (made out of the monomers called amino acids). Amino acids are bonded together via peptide bonds to form a polypeptide known as a protein. It is made up of the elements Carbon, Oxygen, Nitrogen, and Hydrogen. Proteins have a wide variety of functions: they can be enzymes, like amylase; be used in structure, like keratin and collagen; used in transport, like aquaporins and hemoglobin; cell communication, like hormone signals, neurotransmitters, and receptors; defense, like antibodies; movement, like actin and myosin. We'll get into those later. But first, let's cover amino acids.
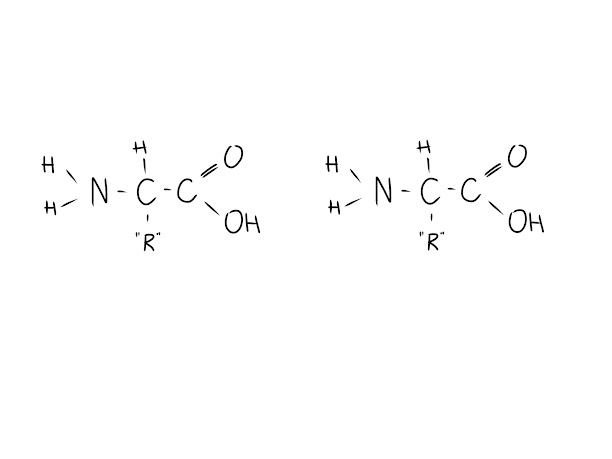
Amino acids look like this:
The NH2 group is the amino group, and the COOH group is the acid group (also referred to as the carboxyl group), connected by a central carbon to a hydrogen and an "R" group (which stands in for anything attached).
What distinguishes each amino acid (there are 20 of them) is the "R" group attached to the central carbon. Each "R" group has unique properties that differentiates it from other amino acids.
But how are amino acids bonded together to form a protein?
Take two amino acids and put them so that the amino group is facing the acid group, as shown.
In a process called dehydration synthesis, the OH from the acid group and the H from the amino group will bond together to form water (H2O). However, they cannot stay bonded to their respective amino acids, because there are now too many bonds; H can only form one bond because it only has one valence electron (outermost orbiting electron). When it bonds to form water, it is now bonded twice - once to water, and once to the amino group. This is physically (chemically) impossible!
So, the water breaks away from the amino acids. But this now poses a different problem: both nitrogen and carbon are missing a bond and need to bond with something to become stable again!
The solution is for the nitrogen and carbon to bond to each other. This way, they're both stable — and look! We've created a polymer.
So, dehydration synthesis is removing a water molecule (dehydration) in order to fuse both amino acids together (synthesis via peptide bonds). It is an anabolic (constructive) reaction and is usually endergonic/endothermic, meaning it requires heat/energy to be put in to happen.
But what if we want to break our freshly made polymer back into two amino acids? We essentially do dehydration synthesis but in reverse.
If removing a water will fuse them together, adding a water will break them apart. This process is called hydrolysis: breaking down a compound using water. It is a catabolic (deconstructive) reaction and is usually exergonic/exothermic, as it releases heat/energy.
Let's move onto polymer structure.
Protein Structure
There are 3 levels of folding these polymer can undergo. The final folding will turn it into a functional protein.
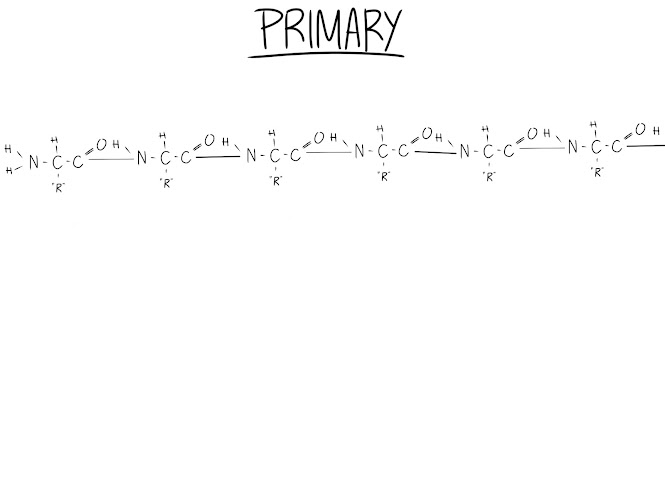
Primary Structure (SEQUENCE):
This is essentially just a sequence of amino acids in a line. The sequence is decided by a gene. We'll cover genes later.
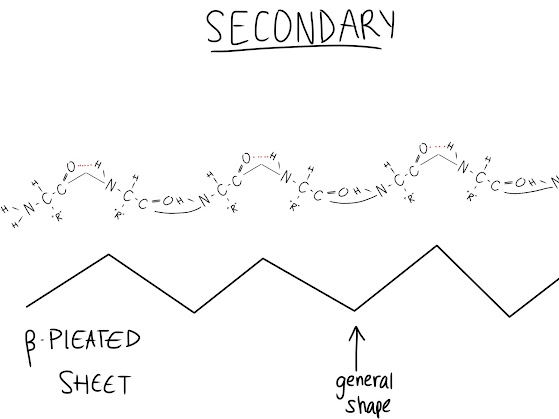
Secondary Structure (COILING VIA H-BONDS):
The first folding stage; interactions are starting to occur between amino acids. Weak hydrogen bonds are starting to form between amino acids and the backbone (the backbone is the long line of N-C-C-N-C-C-N etc. that you get when you combine many amino acids together). The H of the amino group and the O from the acidic group are weakly attracted to each other and will try to move closer together. Two types of structures can be made here: Alpha Helices and Beta-Pleated Sheets.
Tertiary Structure (FOLDING VIA R-GROUPS):
The whole molecule is folding. Interactions between "R" groups of distant amino acids occur. This might be a bit confusing at first because quite a few bonds are now forming. We'll cover this in another unit, but for now, all you need to know is that these proteins are made in the cytoplasm of a cell, and the cytoplasm is water-based, meaning it is POLAR. Therefore, an amino acid that is non-polar will be repelled by the water surrounding it and curl up into itself, while polar amino acids will be attracted by the water and will not curl inwards as much. During the tertiary stage, we will have hydrogen bonds, Van Der Waal's forces, ionic bonds, and disulphide bridges forming. Disulphide bridges are bonds that occur only between two sulfur atoms.
Quaternary Folding (2+ POLYPEPTIDES):
Two or more polypeptide chains bond together via hydrogen bonds. From this point on, the polymer is considered a protein and will be able to do work (by becoming an enzyme or the like).
Now that we've created a nice protein, let's break it back down!
.... but it's stable now, thanks to the numerous bonds holding it together. How do we break it apart?
Denaturation is the process of changing the structure of a protein by disrupting its H-bonds, Van Der Waals bonds, ionic bonds, and disulphide bridges. We can achieve this by changing:
Temperature: increasing the temperature will break weak bonds.
pH level: an increase or decrease in pH will break hydrogen bonds.
Salinity: a change in salinity will break ionic bonds.
Radiation: exposure to radiation will break bonds.
By doing so, we're affecting the secondary, tertiary, and quaternary structures of the protein. This destroys the functionality of the protein. After removing these conditions, the protein may be able to reshape and re-bond itself back into a functioning protein, but also might not.
Chaperone proteins provide a sort of shelter for polypeptides undergoing folding and keeps the new protein segregated from cytoplasmic influences. No, they don't actually look like this, but for the purpose of understanding the concept, we'll pretend they do.
Phew! That was a long lesson. You've earned a joke!
What do you call a couple of chimpanzees sharing an amazon prime account?
PRIME-mates!
(haha)









S H A Z A M
ReplyDeleteIf you denature a protein using every single method (salinity, radiation, pH level) would it break into separate atoms?
ReplyDeleteNope! All those methods of denaturation would definitely destroy the shape of the protein but wouldn't break it into separate atoms. Hydrolysis would be required for this to happen.
Delete